Introduction: Bispecific antibodies (BsAbs) were recently approved for patients with relapsed/refractory (R/R) large B-cell lymphoma (LBCL) and follicular lymphoma (FL) based on phase I-II clinical trials. These T-cell engaging agents achieve 40-50% of complete responses, but information regarding prognostic factors is scarce. Given the deleterious effect of previous bendamustine (benda) reported for patients receiving chimeric antigen receptor (CAR) T-cell therapy, we aimed to assess if prior benda exposure had an impact on BsAb outcomes.
Methods: We conducted a retrospective, multicenter study including patients with LBCL or FL treated at 5 centers with BsAbs from November 2017 to March 2023. We analyzed baseline characteristics, response rates and survival outcomes according to prior benda exposure. Then, we carried out subgroup analyses according to histology (LBCL and FL) and treatment partner (monotherapy and combination regimens), assessing the impact of benda in each of these cohorts. Finally, we examined the impact of benda dose and washout before BsAb start on efficacy outcomes.
Results: We included 143 patients (104 LBCL, 39 FL) who received BsAb therapy, 43 (30%) of which had been previously exposed to benda at a median of 653 days (range 31-2369 days). The BsAb targeted CD20/CD3, either in monotherapy (60%) or in combination with other agents (40%). Median follow-up for the full data set was 21.8 months (CI 95% 18.1 - 25.1 months).
In terms of baseline characteristics (table) median age was 66 years and most patients had an advanced stage of disease (86%), increased LDH (65%) and at least 1 prior line of therapy (90%, median 2 [range 0-9]); CAR T-cell therapy was a prior line for 15% of patients. The benda-exposed cohort had an increased rate of transformed indolent lymphoma (47% vs 25%, p=0.01) and more prior lines of therapy (>2, 53% vs 26%, p<0.01) in comparison with benda-naïve patients.
First, we analyzed the impact of previous benda on several parameters. Patients with prior exposure to benda had lower median CD3+ cells (543 vs 930/mm3, p=0.01) than benda-naïve patients. In terms of toxicity, there were no differences in the incidence or severity of cytokine release syndrome and neurotoxicity. Regarding efficacy, overall and complete response rates (ORR [CRR]) were comparable between benda-exposed and naïve patients (75% [60%] vs 72% [56%], respectively [p=0.73]).
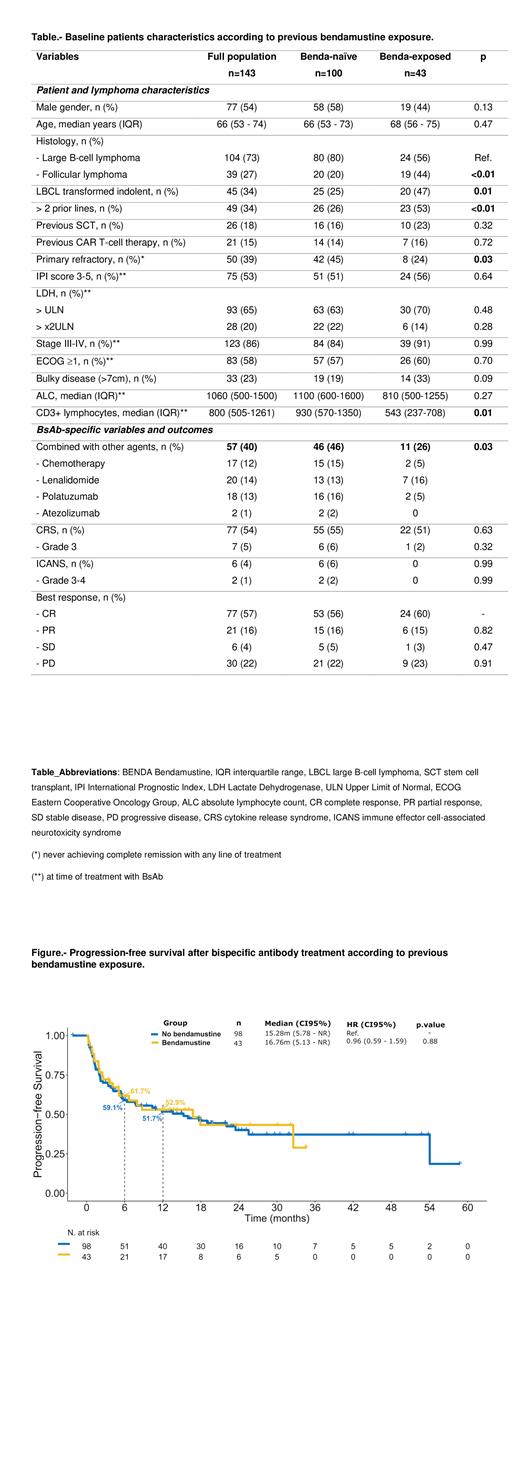
Median PFS and OS for the full cohort were 16.0 months and 43.4 months, respectively, without significant differences for the benda-exposed and naïve cohorts (16.8 vs 15.3 months [p=0.88] and NR vs 43.4 months [p=0.99]). In the univariate analysis for PFS, primary refractory and bulky disease, increased LDH and >2 previous lines were associated with a shorter survival. The same factors carried a negative impact for OS, with the addition of previous CAR T-cell therapy and ECOG >0.
Then, we carried out subgroup analyses according to underlying histology. In the LBCL cohort (n=104), overall and complete response rates were similar between benda-exposed and naïve patients (74% [52%] vs 71% [55%], p=0.81), as were PFS (p=0.78) and OS (p=0.76). In the FL cohort (n=39), overall and complete response rates were also comparable (77% [71%] vs 78% [61%], p=0.94) as were PFS (p=0.96) and OS (p=0.96).
We focused next on the presence of a treatment partner with the BsAb. Overall (complete) response rates were significantly increased in the combination cohort (mainly with immunotherapy [70%] or chemotherapy [30%]), compared to monotherapy (90% [75%] vs 62% [46%], p<0.01), as were 12-month PFS (77% vs 36%, p<0.01) and 12-month OS (88% vs 55%, p<0.01). Inside both of these cohorts, we did not identify any differences in response rate or survival between the benda-exposed and naïve patients.
Given the wide range in washout from last benda dose, we analyzed the impact of this interval on efficacy outcomes. We did not identify any difference according to time between last benda dose and BsAb start. Noteworthy, only 7 patients had a washout shorter than 6 months. In terms of the benda dose, median number of cycles was 6 (range 1-10). We did not identify an impact of benda cycles on response rate or survival.
Conclusions: Bendamustine-containing regimens do not seem to confer a negative prognostic impact before BsAb therapy, in contrast to what has been reported in the CAR T-cell setting. Longer follow-up and larger cohorts are warranted to confirm these results.
Disclosures
Iacoboni:Celgene/Bristol-Myers Squibb: Consultancy, Honoraria; AstraZeneca: Honoraria; Gilead Sciences: Consultancy, Honoraria; Janssen: Honoraria; MSD: Honoraria; Novartis: Consultancy, Honoraria; Miltenyi: Consultancy, Honoraria; Autolus: Consultancy; Abbvie: Honoraria. Lopez Garcia:Beigene: Consultancy; Roche: Consultancy, Speakers Bureau; Janssen: Consultancy, Speakers Bureau; Astrazeneca: Consultancy, Speakers Bureau. Sureda Balari:MSD: Research Funding; Kite: Consultancy, Speakers Bureau; Takeda: Consultancy, Honoraria, Speakers Bureau. Cordoba:Fundacion Jimenez Diaz University Hospital: Current Employment; F. Hoffmann-La Roche Ltd, Takeda, Abbvie, Janssen, AstraZeneca, Lilly, BeiGene, BMS, Genmab, Incyte, Gilead: Consultancy; F. Hoffmann-La Roche Ltd, Takeda, Abbvie, Janssen, AstraZeneca, Lilly, BeiGene, BMS, Genmab, Incyte, Gilead: Speakers Bureau; European Hematology Association (EHA), Spanish Society Hematology (SEHH): Membership on an entity's Board of Directors or advisory committees. Canales:Takeda: Consultancy; Roche: Consultancy; Lilly: Consultancy; Kyowa: Consultancy; Kite: Consultancy; Karyopharm: Consultancy; Janssen: Consultancy; Incyte: Consultancy; BMS: Consultancy; Beigene: Consultancy; Incyte: Speakers Bureau; Janssen: Speakers Bureau; Kite: Speakers Bureau; Kyowa: Speakers Bureau; Roche: Speakers Bureau; Takeda: Speakers Bureau. Barba:Nektar: Consultancy, Membership on an entity's Board of Directors or advisory committees; Amgen: Consultancy, Membership on an entity's Board of Directors or advisory committees; Allogene: Consultancy, Membership on an entity's Board of Directors or advisory committees; BMS: Consultancy, Membership on an entity's Board of Directors or advisory committees; Jazz Pharmaceutical: Consultancy, Membership on an entity's Board of Directors or advisory committees; Incyte: Consultancy, Membership on an entity's Board of Directors or advisory committees; Miltenyi Biotech: Consultancy, Membership on an entity's Board of Directors or advisory committees; Pierre-Fabre: Consultancy, Membership on an entity's Board of Directors or advisory committees; Novartis: Consultancy, Membership on an entity's Board of Directors or advisory committees; Kite/Gilead: Consultancy, Membership on an entity's Board of Directors or advisory committees.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal